Abstract
Background: A large number of tyrosine kinase inhibitors (TKI) discontinuation studies has shown that about 50% of chronic myeloid leukemia (CML) patients (pts) after TKI cessation lose a major molecular response (MMR) and should return to therapy. Despite the fact that, after TKI resumption, almost all patients re-achieve deep molecular remission, facilitating a more accurate selection process for therapy cessation remains topical.
Aim: Develop a prognostic model for the better selection of CML patients for TKI discontinuation.
Methods: The base for the training set was the Russian multicenter prospective study RU-SKI on the discontinuation of TKI in pts with CML and deep molecular response (DMR). Ninety-eight CML pts with chronic phase (CP), TKI therapy for at least three years and a stable DMR (BCR-ABL<0.01%) for at least two years were enrolled. Seven Pts with a previous history of unsuccessful treatment-free remission (TFR) were excluded from the analysis. The BCR-ABL level was evaluated by RQ-PCR according to the international scale (IS). The schedule of molecular tests was as follows: monthly during the first six months (mo) after TKI cessation, every two mo from six to 12 mo and every three mo thereafter. Treatment by the same TKI was resumed in case of MMR loss (BCR-ABL>0.1%). We used the Kaplan-Meier method for calculating the probability of TFR. Univariate analyses were performed using the log-rank test to identify prognostic factors for TFR. Variables found to be significant at the p<0.10 level were entered into a proportional hazards regression analysis. We categorized each independently significant factor into two groups, depending on the optimal cutoff level obtained by ROC analysis and the minimum P-value approach. The next step was a second multivariate analysis including categorized variables. A favorable factor from each variable was scored as 1, while the adverse factor was scored proportionally to the level of hazard ratio. The cumulative score for each patient was calculated. Patients were allocated to either a high or a low risk group concerning MMR loss after the cutoff level was determined by ROC analysis. The validation set included a series of 48 retrospective cases of discontinued patients selected according to RU-SKI inclusion criteria.
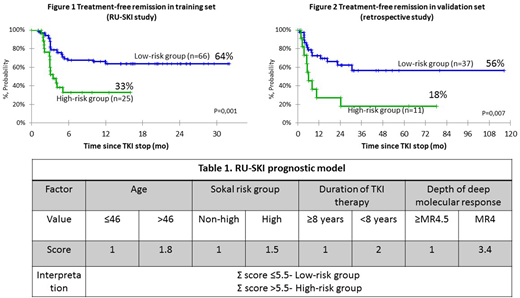
Results: Baseline characteristics of the training set (n=91): male: 48%; median (Mе) age at TKI cessation 46 years (range 22 to 80); Me duration of TKI therapy 8.3 years (range three to 16.2); Me duration of DMR 3.2 years (range two to 10.7). Therapy before treatment cessation: imatinib in 63 (69%) pts, second-generation (2G) TKI in 28 (31%) pts. Me follow-up time after TKI cessation was 14 mo (range three to 36). Probability of TFR was 55% after 12 mo of follow-up. We analyzed the following factors: age, gender, history of previous resistance to imatinib, type and line of TKI, duration of therapy, length of DMR, depth of molecular response before cancellation, Sokal risk group. Age, Sokal risk group, duration of therapy and depth of molecular response were found to be independently significant factors and included in the survival prognostic model (Table 1). 73% (n=66) of pts scored 5.5 points or less and were assigned to the low risk group. The probability of TFR was 64% and 33% for the low risk and high risk groups, respectively (p=0.001) (Figure 1). Baseline characteristics of validation set (n=48): male: 36%; Ме age at TKI cessation 46 years (range 22 to 76); Me duration of TKI therapy six years (range 3.5 to 13.2); Me duration of DMR 2.8 years (range two to 10). Therapy before treatment stopped: imatinib in 31 (65%) pts, 2G TKI in 17 (35%) pts. Me follow-up time after TKI cessation was 36 mo (range six to 116). Probability of TFR was 47% after 30 mo of follow-up. In the validation set, 77% (n=37) of pts were assigned to the low risk group. The probability of TFR was 56% and 18% for the low risk and high risk groups, respectively (p=0.007) (Figure 2).
Conclusion: RU-SKI prognostic model is effective in prediction of successful TFR and can be used for better selection of CML pts for TKI discontinuation.
Shukhov:Novartis: Other: provided consultations and performed lectures ; Bristol Myers Squibb: Other: provided consultations and performed lectures . Chelysheva:Novartis: Other: provided consultations and performed lectures; Fusion Pharma: Other: provided consultations ; Bristol Myers Squibb: Other: provided consultations and performed lectures. Turkina:Novartis: Other: provided consultations; Bristol Myers Squibb: Other: provided consultations; Phizer: Other: provided consultations; Fusion Pharma: Other: provided consultations.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal